Alzheimer’s Dementia and Longevity: H2 Global Group Offers Solutions, Opens an Investment Window, and Targets the Medicine of the Future
Prague, 19 December 2025 (PROTEXT) – Dementia and Alzheimer’s disease are rapidly becoming one of the greatest health, social, and economic challenges of today—and especially of the coming decades. Alzheimer’s dementia is often referred to as the “cancer of the mind.” In its early stages, it does not progress dramatically from day to day, but rather gradually strips a person of memory, identity, and self-sufficiency. The impact affects not only patients, but also their families, healthcare systems, and public budgets.
According to data from the World Health Organization, approximately 57 million people worldwide were living with dementia in 2021, with nearly 10 million new cases added each year. Alzheimer’s disease accounts for roughly 60–70% of all dementia cases. At the same time, the economic burden is rising sharply. Alzheimer’s Disease International reports that global dementia-related costs now exceed USD 1.3 trillion annually and could reach as much as USD 2.8 trillion per year by 2030. This is a problem that affects virtually every country—and therefore all of us.
This is precisely where the topic of dementia intersects with the phenomenon of longevity. The desire to live longer alone is not enough. True longevity means extending the healthy years of life, and above all preserving a young mind—memory, focus, and the ability to make independent decisions. At this point, preventive longevity practice naturally connects with one of the greatest medical challenges of our time.
This is where the Czech group H2 Global Group enters the scene, building a hydrogen-based platform that connects longevity with medicine. “We are not a pharmaceutical giant with an unlimited budget. We have a unique, patent-protected, and scalable approach that brings new possibilities into medicine, which has already attracted the attention of global players. We chose a financing path that also opened space for smaller investors to participate in the growth of the project’s value in the period before key clinical milestones,” says David Maršálek, Founder and CEO of H2 Global Group.
Longevity Boom: One of the Largest Market Potentials of Our Time
The longevity market is currently experiencing an unprecedented investment boom. Estimates suggest that the global longevity market could reach a value of USD 1.87 trillion by 2034. Investors are channeling capital into premium longevity and medical centers, biotechnology focused on aging processes, and concierge medicine, i.e., high-end private care. The common denominator of successful projects is a combination of clinical credibility, measurable and visible results, and a scalable business model. It is precisely within this framework that H2 Global Group is building its hydrogen business as a bridge between the rapidly growing longevity market and traditional medicine—extending all the way to the medicine of the future.
H2 Longevity as a New Standard: Prevention, Performance, and Healthy Aging
The concept of longevity today represents a shift in how individuals, clinics, and service providers approach health: instead of “putting out fires,” the emphasis is on long-term vitality, regeneration, resilience of the body, and overall quality of life. H2 Global Group has embraced this trend in a practical way, creating a scalable model for end customers and B2B partners, while connecting it with technologies and know-how in the field of molecular hydrogen and its safe practical application. The company’s clients already include a wide range of prominent partners—from athletes and sports clubs, spa facilities, and private clinics including dental practices, to wellness hotels and centers focused on biohacking, longevity, and overall regeneration of the body.
H2 Longevity® Lifestyle Ostrava: A New-Generation Center Not Only for Prevention
One of the most visible steps this year was the opening of H2 Longevity® Ostrava, publicly presented as a new-generation healthy longevity center and at the same time as a model space (“showroom”) for customers, distributors, professional partners, and investors.
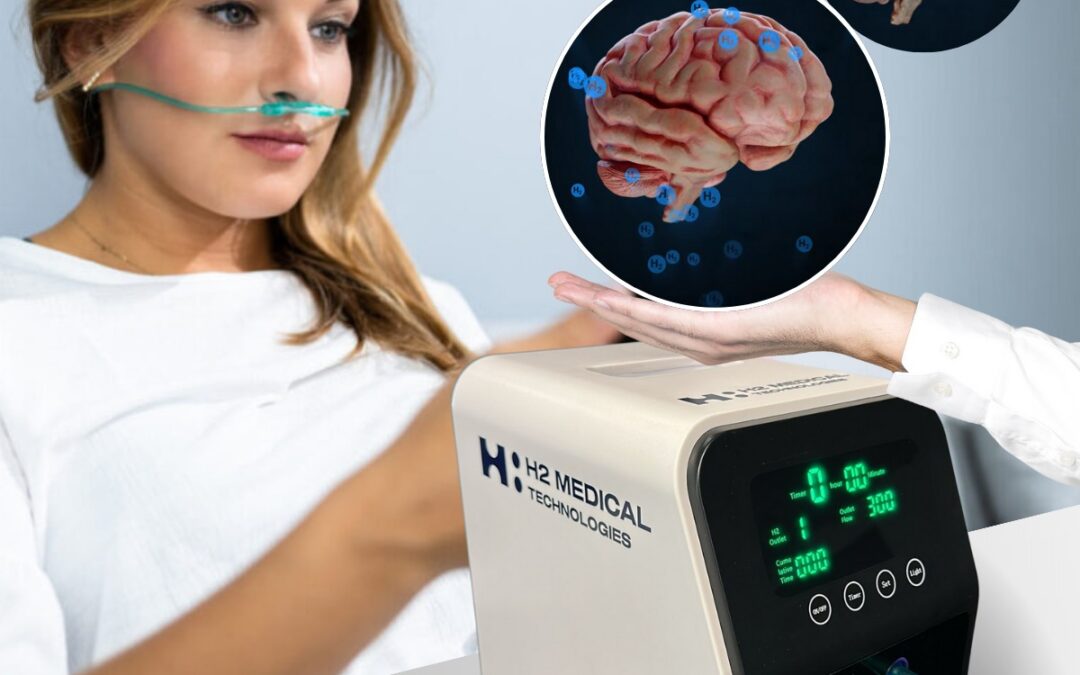
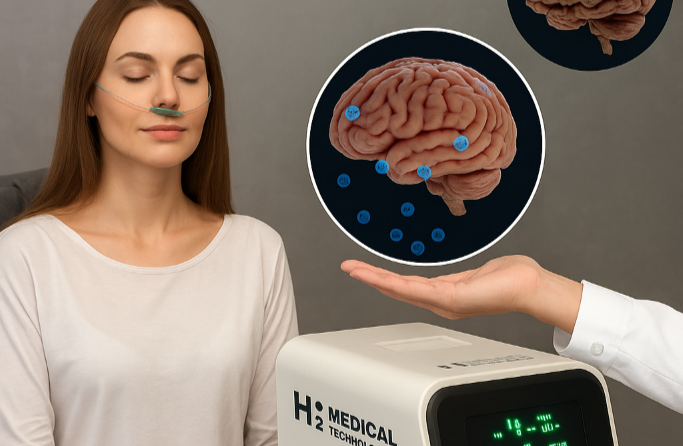
The center combines modern longevity practices such as molecular hydrogen inhalation using H2 Medical Technologies devices, light therapy, hydrogen hydration in the form of H2 Premium® hydrogen water, specialized H2 dietary supplements such as H2 Brain® and H2 Forte®, and unique hydrogen-based cosmetics, including education focused on regeneration and healthy aging. The Ostrava center thus serves not only as a premium destination for end clients, but also as a reference point for future expansion through partners.
From Longevity to the Medicine of the Future Through an Approved Clinical Trial
A key step is the transition from prevention to data-driven medicine. Through its MedTech division, H2 Medical Technologies, H2 Global Group announced that the Czech State Institute for Drug Control (SÚKL) has approved the first clinical study in the Czech Republic to evaluate the safety of molecular hydrogen inhalation in patients with mild cognitive impairment (MCI), a condition often considered a precursor to Alzheimer’s dementia. The study will use the first functional prototype of a molecular hydrogen-generating device developed by H2 Medical Technologies.
The study will begin in January 2026, last six months, and include approximately 30 patients aged 50–80, followed by a monitoring phase and data evaluation. Candidates still have the opportunity to apply for participation (see below). The approved clinical study is followed by another strategic ambition of the group: the registration of the world’s first medical device utilizing molecular hydrogen. The initial focus is on the use of molecular hydrogen in Alzheimer’s dementia (both prevention and treatment), with potential expansion into other indications such as type 2 diabetes, vision and hearing disorders, skin conditions, and more. This modularity is also important for the partner ecosystem, allowing clinics and longevity service providers to gradually expand their offerings and target specific client needs—from regeneration and vitality to specialized supplementary protocols.
Call for Participation in the Clinical Study
H2 Medical Technologies has also issued a public call for volunteers who wish to participate in the study and meet the basic criteria: age 50–80, overall good health, a diagnosis of mild cognitive impairment, or mild to moderate dementia, and ideally residence in Ostrava or the surrounding area where the study will take place. Participation in the study does not guarantee any health benefit and should not be considered a substitute for standard treatment. Interested individuals can apply by 19 January 2026 via email: david.skoloudik@H2medical.com
A Patent That Turns a Molecule into a Platform
H2 Global Group continues to systematically build its intellectual property portfolio and follows up on previously registered patents with a newly granted European patent EP3701956B1, “Prophylactic or Therapeutic Agent for Dementia,” originated by Japanese Professor Shigeo Ohta and his team, with H2 Global Group as the patent holder. The patent describes the use of molecular hydrogen in neurodegenerative diseases, including dementias. The patent is currently in the process of being extended to additional global territories. From an investment perspective, the key factor is the combination of scientific foundations, patent protection, a regulatory plan, and clinical data—through which a “simple molecule” becomes a scalable technological platform.
The Path to Regulated Medicine: Clinical Data, Safety, and Responsibility
Professional leadership toward regulated medicine is overseen by PharmDr. Milan Krajíček, Director of Research and Development at H2 Medical Technologies. “Our solution represents a completely new, non-invasive, and safe method, whose effects on neurodegenerative diseases are now the subject of clinical testing,” he says. With regard to the launch of the study, he adds that initiating a clinical trial directly linked to Professor Ohta’s patent is both an honor and a responsibility. For investors and clinics, this is a clear signal that the goal is not a marketing story, but a medically tangible project grounded in data. Safety is paramount. Neurologist Prof. MUDr. David Školoudík, Ph.D., the medical guarantor of the study, states: “The device produces hydrogen at a relatively low and safe concentration.”
From a Personal Story to an International Ambition
David Maršálek is now successfully leveraging his many years of practical experience and studies at Dublin Business School in Ireland to build an international technological platform in the fields of longevity and modern medicine. Fourteen years have passed since he met Professor Dušan Miljkovič in the United States—a scientist and his first mentor, who was the first in the world to introduce a hydrogen-based dietary supplement. This moment sparked a journey that gradually evolved into a project with global ambitions.
Maršálek has built a team of experts he collaborates with, including Professor Shigeo Ohta from Japan, a team from Palacký University led by Associate Professor Michal Botek, PharmDr. Milan Krajíček, MUDr. Pavel Malovič, PhD., MPH, and others. The result of this long-term collaboration is the creation of H2 Global Group and H2 Medical Technologies, which today focus on research and development of molecular hydrogen in medicine.
Virtual Reality with Molecular Hydrogen: A Globally Unique Ecosystem
H2 Global Group has also expanded its ecosystem into rehabilitation and psychology through a 20% equity stake in VR LIFE / VR Vitalis. This project is now among the most advanced digital rehabilitation systems in Europe and, following H2 Global Group’s entry, has shown rapid and measurable growth: the number of therapeutic modules expanded from 22 to 52, the solution obtained MDR certification as a medical device, and it is actively used in clinical practice across the European Union. To date, more than 63,000 therapeutic exercises have been conducted on the VR Vitalis platform, confirming not only technological maturity but also a high level of acceptance by therapists and patients. The system is deployed in hospitals and specialized facilities in the Czech Republic, Slovakia, and other countries, and today ranks among the three most widely used therapeutic VR platforms in Europe in terms of real clinical application.
The uniqueness of this project within the H2 Global Group ecosystem lies in the integration of regulated VR rehabilitation with molecular hydrogen inhalation, protected by a utility model and a patent application. This combination makes it possible to simultaneously work with attention, mental calming, guided breathing, and physiological processes associated with cellular regeneration, including in the brain. “Virtual reality allows the patient to fully immerse themselves in a guided environment, disconnect from external stressors, and focus on breathing and movement. When this state is combined with molecular hydrogen inhalation, a very interesting space emerges for supporting mental balance, rehabilitation, and regeneration,” explains Mgr. Jana Trdá, Ph.D., co-founder of VR LIFE.
For partners, this platform represents another practical and immediately usable level of cooperation—from rehabilitation and neurological centers to private clinics, sports medicine, and preventive and longevity programs. From both a business and clinical perspective, it is a unique example of how regulated medical technology, real-world data, and a scalable model with global potential can be combined.
Investment Window Before Reaching Key Milestones
In MedTech, company value typically does not increase linearly, but rather in jumps—at moments when key milestones are achieved, such as the launch of a clinical trial, data evaluation, or successful regulatory steps. These moments often transform a project from a technological story into a strategic asset sought after by global players.
H2 Global Group openly communicates its ambition to achieve registration of the world’s first medical device utilizing molecular hydrogen, so that it becomes available within healthcare reimbursement systems in medical facilities and to the general public, and subsequently to expand the platform with additional applications and indications. The fact that such regulatorily anchored platforms become acquisition targets worth tens of billions of dollars is confirmed by long-term market developments: Medtronic acquired Covidien for approximately USD 43 billion, Abbott acquired St. Jude Medical for about USD 25 billion, Boston Scientific acquired Guidant for USD 27 billion, BD bought CR Bard for USD 24 billion, and Johnson & Johnson paid around USD 20 billion for Synthes and another USD 17 billion for Abiomed. These transactions clearly show that extraordinary value in MedTech arises when clinical data, regulatory credibility, a scalable business model, and strong market positioning come together. These are precisely the factors H2 Global Group is deliberately focusing on at this stage. This is where an attractive entry opportunity opens today for investors who want to enter before key clinical and regulatory milestones—at a point with historically the highest potential for appreciation.
A Strategic Call for Investors and Partners: Enter a New Era of Longevity and the Medicine of the Future
H2 Global Group now stands at the intersection of several fundamental global trends: the rapidly growing longevity economy, the dramatically increasing problem of dementia, and the need for new, measurable, and safe medical solutions that can withstand clinical and regulatory scrutiny. For investors and partners, a period is opening that can be compared to historic moments when humanity found answers to major health threats—from the discovery of antibiotics to breakthrough vaccination and therapeutic platforms. In each case, it was not just about a scientific discovery, but about the emergence of entirely new industries and long-term market leaders. A similar chapter has already begun in the field of cognitive longevity and the use of molecular hydrogen in medicine.
“We process new inquiries daily not only from investors, but also from potential partners from various parts of the world who see an opportunity to expand their portfolios or activities with our hydrogen solutions,” says David Maršálek. “H2 Global Group is therefore deliberately opening space for investors, distributors, and strategic partners who want to co-create a new standard of longevity and the medicine of the future, and to be present at the birth of this unique platform before its value is fully reflected in the global market,” concludes David Maršálek.
Company contacts:
www.H2Global.group
www.H2invest.cz